Products are produced in control workshops, including 100000 level purification workshops and 300000 level control workshops.
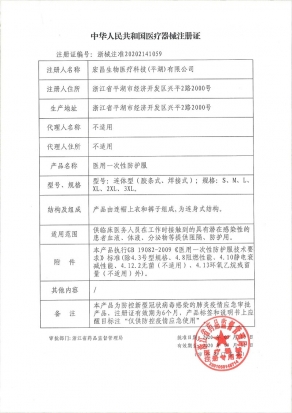
ISO13485:2016,ISO11135:2014,CEauthentication,Medical device production license, domestic Class II product registration certificate, such as disposable surgical uniforms, disposable surgical towels and bags; Domestic Class I product registration certificate, such as isolation clothing, medical isolation shoe covers, etc.